INTRODUCTION
In February 2020, the World Health Organization (WHO) reported the rapidly spreading global outbreak of a new (novel) coronavirus disease, COVID-19, that had appeared in the last part of 2019 in the city of Wuhan, China. WHO announced that COVID-19 was a pandemic that was an international threat and a global public health emergency1. Over the last two years, this pandemic has caused significant changes in the daily life of people, especially in education and working conditions, in addition to mortality and morbidity rates. During this period, significant progress has been made in the fight against the pandemic with effective health policies, lifestyle changes and widespread vaccination programs. However, especially due to the emergence of new variants, COVID-19 has still not been fully controlled. Efforts to control the pandemic have caused unprecedented economic and social disruption all over the world2,3.
The immune system of the pregnant woman, the changes in her cardiopulmonary, respiratory and physiological systems put her at high risk in terms of becoming infected with respiratory viruses and developing more severe disease. Studies conducted so far have not provided any evidence that pregnant women are any more susceptible to COVID-19 than others4–6. However, the course of disease seems to be worse in pregnant women compared to non-pregnant women of the same age. Although more than 90% of pregnant women with COVID-19 recover without serious morbidity, rapid deterioration of disease may be observed; especially symptomatic pregnant women have a higher risk for severe disease compared to the symptomatic non-pregnant women with COVID-192,7,8.
Current studies to date have shown that pregnant women with COVID-19 are at higher risk for serious illness, need for mechanical ventilation, admission to the intensive care unit, and maternal death. In addition, obstetric complications such as preeclampsia, preterm birth, and premature rupture of membranes, fetal distress, and stillbirth are more common in pregnancies complicated by COVID-199,10. Moreover, some recent publications show that pregnant women infected with new SARS-CoV-2 variants have a worse prognosis11,12. In a study comparing pre-variant and post-variant pregnant groups in Turkey, it was reported that there was a significant increase in the rates of serious and critical cases and in the rates of pregnancy complications, preterm delivery, respiratory support, intensive care unit admission and maternal death, and admission to NICU in the post-variant group3. Although there is evidence of intrauterine transmission of SARS-CoV-2, it is reported to occur rarely2.
Health professionals have an important responsibility in the periods of pregnancy, birth and the postpartum, not only to provide follow-up and care for both mother and child but also to offer preventive, early diagnosis, treatment and individualized care services13-15. Vaccination appears to be one of the most effective means of protection in the COVID-19 pandemic. COVID-19 vaccines approved by the U.S. Food and Drug Administration can be administered to pregnant or lactating women2. In the management process of the COVID-19 pandemic, health professionals need to manage the perinatal period more effectively, using a range of comprehensive knowledge to protect and improve maternal and infant health. They can carry out their follow-up and care services in the perinatal process in accordance with the guidelines published by both national and international sources13-15, thus contributing to the production of more comprehensive scientific knowledge.
During the planning and execution of this study, a look into the literature brings forth systematic reviews, case reports, case series and retrospective cross-sectional international studies conducted with small samples that report perinatal outcomes for pregnant women with COVID-19. However, two systematic review studies have recently been published covering approximately the same time as our study. In these systematic reviews, important information about the effects of COVID-19 infection on mother–infant health has been revealed9,10. On the other hand, there is still an urgent need for high quality evidence-backed scientific studies that will disclose broader sets of data on the subject. It was for this reason that this systematic review and meta-analysis was conducted to examine the maternal and infant health outcomes of pregnant women with COVID-19 during the pregnancy, birth and postpartum periods.
METHODS
Protocol and registration
The protocol of this systematic review and meta-analysis was registered on the PROSPERO database (CRD42020191106; registered 11 June 2020). The review and its reporting were followed up on PRISMA (Preferred Reporting items for Systematic Review and Meta-Analysis)16. To keep the risk of bias under control during the study, the scanning, selection of articles, data extraction as well as the quality assessment of the articles included were handled independently by two researchers. Whenever a difference of opinion came up with regard to any aspect of the study, all of the researchers held a discussion session together and arrived at an agreement. At the same time, prior to the start of the study, a pilot study that included all the stages of the research was conducted with the participation of all of the authors, who concurred on a common road map.
Eligibility criteria
The research included those studies published in English between 1 December 2019 and 15 May 2020 and whose full texts could be accessed. The PICOS criteria were considered in the selection of the studies that would be suitable for this systematic review and meta-analysis (Table 1). The exclusion criteria encompassed traditional and systematic reviews and letters to the editor that did not refer to specific cases.
Table 1
PICOS criteria for inclusion of studies and data extraction
Searching strategy
The scanning for this systematic review and meta-analysis was carried out over the period 5–15 May 2020 independently by two of the researchers. Scanning for the international studies was carried out in the PubMed, Ovid, Science Direct, EBSCO and Web of Science, Google Scholar electronic databases with the search string: [‘COVID-19’ AND (‘pregnancy’ OR ‘pregnant’ OR ‘maternal outcomes’ OR ‘infant outcomes’ OR ‘fetal outcomes’ OR ‘birth’)]. At the same time, additional studies were independently checked by the other two authors against the included articles and the reference lists for the review studies.
Selection of studies
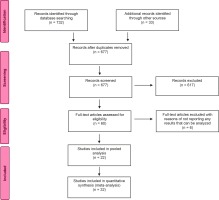
Through the processes of scanning, selection according to heading and/or abstract, and the elimination of repetitious articles. The four authors then met together to decide upon the studies that could be taken into the analysis on the basis of full texts. Later, some studies were eliminated from the scope of the analysis during the extraction or analysis processes because they did not contain data appropriate for the analysis. The selection process for the articles can be seen in Figure 1 with a PRISMA flow diagram.
Data extraction
The researchers devised a data extraction instrument to be used in obtaining study data (Table 2). This tool made it possible to collect information on the studies included in the systematic review and meta-analysis in terms of author information, the country and year of publication, data collection dates, design, sample size, maternal age and other main findings reported in the studies (Table 1). In four studies of case-control design, only the data pertaining to the cases (pregnant women diagnosed with COVID-19) were extracted.
Table 2
Characteristics and main findings of the studies included in the systematic review and meta-analyses
| Study and Country | Date of data collection | Study design | Sample size Age (years) | Data on COVID-19 | Data on pregnancy, birth and maternal health | Data on baby’s health | Quality score |
|---|---|---|---|---|---|---|---|
| Alzamora et al.58 Peru | 29 March 2020 | Case report | n=1 44 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestation period (weeks), mode of delivery, preterm delivery | Birth weight, 1 and 5 min APGAR score, intubation NICU admission, IgG, IgM and RT-PCR test outcomes, breastfeeding | Yes: 7/8 |
| Breslin et al.29 USA | 13–27 March 2020 | Retrospective | n=43 Mean: 29.7±6.0 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), preterm delivery, pregnancy-related disorders, mode of delivery, caesarean indications, ICU admission, antepartum and postpartum hospitalization and treatment protocol, explanation of the care process | 1 and 5 minutes APGAR score, NICU admission, prematurity, additional diagnoses, RT-PCR test outcomes, breastfeeding | Yes: 6/8 |
| Browne et al.18 Colombia | - | Case report | n=1 33 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), preterm delivery, pregnancy-related disorders, explanation of the care process | - | Yes: 7/8 |
| Buonsenso et al.19 Italy | - | Case series | n=2 38 and 42 | Symptoms, diagnostic test, treatment protocol | Gestation period (weeks), mode of delivery, explanation of the care process | Birth weight, 1 and 5 min APGAR score, fetal distress, NICU admission, IgG, IgM and RT-PCR test outcomes, breastfeeding | Yes: 9/10 |
| Cao et al.37 China | 23 January – 23 February 2020 | Retrospective | n=10 (Twins: 1) 29 (n=4), 30 (n=3), 31 (n=2), 35 (n=1) | Symptoms, diagnostic test | Gestational period (weeks), preterm delivery, pregnancy-related disorders, mode of delivery, caesarean indications, explanation of the care process | Birth weight, prematurity, 1 and 5 min APGAR score, fetal distress, neonatal asphyxia, neonatal death, RT-PCR test outcomes | Yes: 6/8 |
| Chen et al.38 China | 20–31 January 2020 | Retrospective | n=9 26 (n=3), 27, 28, 29, 33, 34, 40 | Symptoms, comorbidities, diagnostic test | Gestational period (weeks), preterm delivery, pregnancy-related disorders, mode of delivery | Birth weight, low birth weight, prematurity, 1 and 5 min APGAR score, fetal distress, neonatal asphyxia, intrauterine and neonatal death, RT-PCR test outcomes | Yes: 6/8 |
| Chen et al.39 China | 8 December 2019 – 20 March 2020 | Retrospective | n=118 Median 31 (range: 28-34) (Twins: 2) | Symptoms, diagnostic test, treatment protocol | Preterm delivery, maternal death, mode of delivery, caesarean indication, abortion | 1 min APGAR score, neonatal death, neonatal asphyxia; RT-PCR test outcomes | Yes: 6/8 |
| Chen et al.56 China | 20 January – 10 February 2020 | Case series | n=5 25, 29 (n=2), 30, 31 | Symptoms, diagnostic tests | Gestational period (weeks), pregnancy-related disorders, mode of delivery, postpartum health status | Birth weight, 5 min APGAR score, fetal tachycardia, RT-PCR test outcomes, feeding | Yes: 10/10 |
| Chen et al.40 China | 22–28 February 2020 | Case series | n=4 23, 28, 31, 34 | Symptoms, comorbidities, diagnostic test | Gestational period (weeks), pregnancy-related disorders, mode of delivery, explanation of the care process, postpartum health status | Birth weight, 1 and 5 min APGAR score, fetal distress, NICU admission, RT-PCR test outcomes, feeding | Yes: 9/10 |
| Dong et al.59 China | 28 January –28 February 2020 | Case report | n=1 29 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, explanation of the care process | Birth weight, 1 and 5 min APGAR score, NICU admission, IgG, IgM and RT-PCR test outcomes | Yes: 7/8 |
| Fan et al.57 China | 10 January –27 February 2020 | Case series | n=2 29, 34 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, explanation of the care process | Birth weight, 1 and 5 min APGAR score, RT-PCR and Chest CT test outcomes, feeding | Yes: 9/10 |
| Ferrazzia et al.41 Italy | 1–20 March 2020 | Retrospective | n=42 (21–44) | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), pregnancy-related disorders, ICU admission, mode of delivery, caesarean indications, postpartum health status, explanation of the care process | Birth weight, 5 min APGAR score, NICU admission, RT-PCR test outcomes, feeding | Yes: 6/8 |
| Gidlof et al.20 Sweden | - | Case report | n=1 (Twins: 1) 34 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), pregnancy-related disorders, mode of delivery, explanations of the care process | Birth weight, low birth weight, 1 and 5 min APGAR score, NICU admission, RT-PCR test outcomes, feeding | Yes: 7/8 |
| Govind et al.42 United Kingdom | 7 March – 22 April 2020 | Case series | n=9 median: 31 (range: 18–39) | Symptoms, diagnostic test, comorbidities | Gestational period (weeks), preterm delivery, pregnancy-related disorders, mode of delivery, caesarean indications, explanations of the care process | Birth weight, low birth weight, 1 and 5 min APGAR score, NICU admission, mechanical ventilation, RT-PCR test outcomes, feeding | Yes: 9/10 |
| Hantoushzadeh et al.43 Iran | February –March 2020 | Case series | n=9 25–49 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), maternal death, mode of delivery | Birth weight, low birth weight, 1 and 5 min APGAR score, RT-PCR test outcomes, neonatal pneumonia, intrauterine and postpartum infant death | Yes: 10/10 |
| Hirshberg et al.21 United States | - | Case series | n=5 27, 29, 33, 35, 39 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), preterm delivery, mode of delivery, Acute Respiratory Distress Syndrome, ICU admission, mechanical ventilation | Birth weight, low birth weight, 1 and 5 min APGAR score, RT-PCR test outcomes | Yes: 9/10 |
| Iqbal et al.22 United States | - | Case report | n:1 34 | Symptoms, diagnostic test | Gestational period (weeks), mode of delivery, explanations of the care process | 1 and 5 min APGAR score, feeding | Yes: 7/8 |
| Juusela et al.44 United States | March 2020 | Case series | n=2 26, 45 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), pregnancy-related disorders, mode of delivery explanations of the care process, postpartum health status | Fetal tachycardia | Yes: 9/10 |
| Kalafat et al.60 United States | 19–29 March 2020 | Case report | n=1 32 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), preterm delivery, mode of delivery, explanations of the care process, postpartum health status | Birth weight, 1 and 5 min APGAR score, RT-PCR test outcomes | Yes: 7/8 |
| Karami et al.23 Iran | - | Case report | n=1 27 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, ICU admission, explanations of the care process, maternal death | 1 and 5 min APGAR score, intrauterine death | Yes: 7/8 |
| Khan et al.45 China | 28 January – 1 March 2020 | Case series | n=3 27, 28, 33 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), preterm delivery, mode of delivery, explanations of the care process | Birth weight, 1 and 5 min APGAR score, NICU admission, RT-PCR test outcomes, neonatal death | Yes: 9/10 |
| Khan et al.46 China | 25 January – 15 February 2020 | Case series | n=17 Mean: 29.29 (range: 24–34) | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), preterm delivery, mode of delivery | Birth weight, low birth weight, 1 and 5 min APGAR score, RT-PCR test outcomes, neonatal pneumonia, neonatal death | Yes: 10/10 |
| Koumoutsea et al.24 Canada | - | Case series | n=2 23, 40 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, postpartum bleeding, explanations of the care process | Birth weight, low birth weight, 1 and 5 min APGAR score, fetal bradycardia, feeding | Yes: 9/10 |
| Lee et al.61 Korea | 14 February 2020 | Case report | n=1 28 | Symptoms, diagnostic test | Gestational period (weeks), preterm delivery, mode of delivery, cephalopelvic disproportion, explanations of the care process | Birth weight, 1 and 5 min APGAR score, NICU admission, RT-PCR test outcomes, feeding | Yes: 6/8 |
| Li et al.30 China | 24 January – 29 February 2020 | Case-control | n=16 Mean: 30.9±3.2 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), preterm delivery, mode of delivery, pregnancy-related disorders, ICU admission, explanations of the care process, postpartum health status | Birth weight, low birth weight, 1 and 5 min APGAR score, fetal distress, RT-PCR test outcomes | Yes: 7/10 |
| Li et al.62 China | 6 February 2020 | Case report | n=1 30 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, explanations of the care process | RT-PCR test outcomes | Yes: 7/8 |
| Li et al.63 China | 28 January 2020 | Case report | n=1 31 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery | Premature, neonatal death | Yes: 7/8 |
| Liao et al.47 China | 20 January – 2 March 2020 | Case-control | n=10 27, 29 (n=2), 30 (n=2), 33 (n=2), 36 (n=3) | Symptoms, diagnostic test | Gestational period (weeks), preterm delivery, mode of delivery, pregnancy-related disorders, data on birth, explanations of the care process | Birth weight, premature, 1 and 5 min APGAR score, fetal distress, RT-PCR test and chest radiograph outcomes, hyaline membrane disease, neonatal asphyxia | Yes: 7/10 |
| Liu et al.48 China | 20 January –10 February 2020 | Prospective/cross-sectional | n=15 Mean: 32±5 (range: 23–40) | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, pregnancy-related disorders, explanations of the care process, postpartum health status | 1 and 5 min APGAR score, fetal distress, neonatal asphyxia, neonatal death, stillbirth | Yes: 6/8 |
| Liu et al.49 China | 31 January – 29 February 2020 | Prospective/cross-sectional | n=19 Median: 31 (range: 27–34) | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, pregnancy-related disorders, explanations of the care process | Birth weight, 1 and 5 min APGAR score, fetal distress, NICU admission, RT-PCR test and chest radiograph outcomes, feeding | Yes: 6/8 |
| Liu et al.50 China | 2–5 February 2020 | Case series | n=3 30 (n=2), 34 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, explanations of the care process | Birth weight, 1 and 5 min APGAR score, fetal distress, meconium aspiration NICU admission, RT-PCR test outcomes, feeding | Yes: 9/10 |
| Liu et al.51 China | 8 December 2019 – 25 February 2020 | Case series | n=13 22, 24, 26, 28, 29, 30 (n=3), 31, 32, 33, 35, 36 | Symptoms, diagnostic test | Gestational period (weeks), preterm labor, mode of delivery, pregnancy-related disorders, ICU admission, mechanical ventilation, postpartum health status, explanations of the care process | 1 and 5 min APGAR score, fetal distress, stillbirth, RT-PCR test outcomes | Yes: 9/10 |
| Lowe and Bopp25 Australia | - | Case report | n=1 31 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, explanations of the care process | 1 and 5 min APGAR score, RT-PCR test outcomes, feeding | Yes: 7/8 |
| Lu et al.64 China | 11–27 February 2020 | Case report | n=1 22 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, postpartum health status, explanations of the care process | Birth weight, 1 and 5 min APGAR score, fetal distress, RT-PCR and Chest CT test outcomes, feeding | Yes: 7/8 |
| Lyra et al.26 Portugal | - | Case report | n=1 35 | Symptoms, diagnostic test | Gestational period (weeks), mode of delivery, pregnancy-related disorders, explanations of the care process | Birth weight, 1 and 5 min APGAR score, RT-PCR test outcomes | Yes: 7/8 |
| Martinelli et al.28 Italy | 29 March – 14 April 2020 | Case report | n=1 17 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), preterm labor, mode of delivery, caesarean indication | Birth weight, low birth weight, NICU admission | Yes: 7/8 |
| Peng et al.27 China | - | Case report | n=1 25 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery | Birth weight, 1 and 5 min APGAR score, fetal distress, NICU admission, RT-PCR test results | Yes: 7/8 |
| Qiancheng et al.31 China | 15 January – 15 March 2020 | Case-control | n=28 Median: 30 (range: 26.75–32.00) | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), preterm labor, mode of delivery, medical abortion, explanations of the care process | Birth weight, low birth weight, 1 and 5 min APGAR score, neonatal asphyxia, NICU admission, RT-PCR test outcomes, intrauterine death, postpartum infant death | Yes: 7/10 |
| Song et al.65 China | 6 February 2020 | Case report | n=1 30 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery | Birth weight, 1 and 5 min APGAR score, RT-PCR test outcomes, feeding | Yes: 7/8 |
| Wang et al.66 China | 1–18 February 2020 | Case report | n=1 34 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), preterm labor, mode of delivery, pregnancy-related disorders, amniotic fluid with meconium, postpartum health status | Birth weight, 1 and 5 min APGAR score, RT-PCR test outcomes, feeding | Yes: 7/8 |
| Wang et al.67 China | 2–18 February 2020 | Case report | n=1 28 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), preterm labor, mode of delivery pregnancy-related disorders, ICU admission | Birth weight, 1 and 5 min APGAR score, NICU admission, RT-PCR test outcomes, feeding | Yes: 7/8 |
| Wen et al.68 China | 4–20 February 2020 | Case report | n=1 31 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), pregnancy continues | - | Yes: 7/8 |
| Wu et al.52 China | 23 January – 10 February 2020 | Retrospective | n=8 26, 28, 29, 30 (n=3), 31, 35 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), preterm labor, mode of delivery pregnancy-related disorders, ICU admission caesarean indications, postpartum health status | Fetal distress | Yes: 6/8 |
| Wu et al.53 China | 31 December 2019 – 7 March 2020 | Retrospective | n=23 Median: 29 (range: 21–37) | Symptoms, comorbidities, diagnostic test | Gestational period (weeks), mode of delivery pregnancy-related disorders, explanations of the care process | Birth weight, 1 and 5 min APGAR score, fetal hypoxia, RT-PCR test outcomes, clinical diagnostic criteria | Yes: 6/8 |
| Xiong et al.69 China | 29 January – 10 March 2020 | Case report | n=1 25 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery pregnancy-related disorders | Birth weight, 1 and 5 min APGAR score, fetal distress, IgG, IgM and RT-PCR test outcomes | Yes: 7/8 |
| Xu et al.54 China | 21 January – 9 February 2020 | Retrospective | n=5 23, 25, 28, 34 (n=2) | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), preterm labor, mode of delivery, pregnancy-related disorders, caesarean indications, postpartum health status, explanations of the care process | Birth weight, 1 and 5 min APGAR score, fetal distress, NICU admission, RT-PCR test outcomes, intrauterine death, postpartum infant | Yes: 6/8 |
| Yang et al.32 China | 20 January – 5 March 2020 | Case-control | n=13 Mean: 30.2±2.3 | Symptoms | Gestational period (weeks), preterm labor, mode of delivery, pregnancy-related disorders, | Yes: 7/10 | |
| Yang et al.33 China | 20–29 January 2020 | Case series | n=7 | Symptoms, diagnostic test, treatment protocol | Gestational period (weeks), preterm labor, mode of delivery, pregnancy-related disorders, caesarean indications, explanations of the care process | Birth weight, 1and 5 min APGAR score, NICU admission, RT-PCR test outcomes | Yes: 1/10 |
| Yu et al.55 China | 1 January – 8 February 2020 | Case series | n=7 29, 30, 31, 33, 34 (n=3) | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), mode of delivery, ICU admission, explanations of the care process | Birth weight, low birth weight, 1 and 5 min APGAR score, RT-PCR test outcomes | Yes: 10/10 |
| Zamaniyan et al.70 Iran | 7–26 March 2020 | Case report | n=1 22 | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), preterm labor, mode of delivery, ICU admission, maternal death, explanations of the care process | Birth weight, low birth weight, 1 and 5 min APGAR score, postpartum fever, RT-PCR test outcomes, feeding | Yes: 7/8 |
| Zambrano et al.71 Honduras | 9 March 2020 | Case report | n=1 41 | Symptoms, comorbidities, diagnostic test | Gestational period (weeks), preterm labor, mode of delivery, explanations of the care process | Birth weight, low birth weight, fetal anomaly, RT-PCR test outcomes | Yes: 7/8 |
| Zeng et al.34 China | 16 February – 6 March 2020 | Retrospective | n=6 | Symptoms, diagnostic test | Gestational period (weeks), mode of delivery, explanations of the care process | 1 and 5 min APGAR score, IgG, IgM and RT-PCR test outcomes | Yes: 6/8 |
| Zeng et al.35 China | January –February 2020 | Retrospective | n=33 | Symptoms, diagnostic test | Gestational period (weeks), preterm labor, mode of delivery, caesarean indications, pregnancy-related disorders, amniotic fluid with meconium, ICU admission, postpartum health status | Birth weight, prematurity, 1 and 5 min APGAR score, fetal distress, fetal asphyxia, NICU admission, intrauterine death, postpartum infant death, RT-PCR test outcomes, treatment protocol, mechanical ventilation | Yes: 6/8 |
| Zhu et al.36 China | 20 January – February 2020 | Retrospective | n=9 (Twins: 1) 25, 29, 30 (n=4), 34, 35 (n=2) | Symptoms, comorbidities, diagnostic test, treatment protocol | Gestational period (weeks), preterm labor, mode of delivery, explanations of the care process | Birth weight, low birth weight, prematurity, 1 and 5 min APGAR score, fetal distress, RT-PCR test and chest radiograph outcomes, diet, postpartum infant death | Yes: 6/8 |
Methodological quality evaluation of the studies
The checklists drawn up by the Joanna Briggs Institute for cross-sectional studies, case studies, case series and case-control studies were used in the quality assessment of the articles17. The first and second of these tools comprise eight items, while the third and fourth comprise 10 items. Each item is assessed as Yes, No, Uncertain and Inapplicable. The results of the assessment were presented on the basis of the total number of items considered (number of Yes) as a ‘Quality Score’, given in Table 2.
Synthesis of the data
The data in this systematic review and meta-analysis were combined with a meta-analysis and a consolidated percentage calculation. The data obtained from the studies of retrospective and perspective cross-sectional, case series and case-control design were consolidated in the meta-analysis. The data from the case reports were consolidated by pooled analysis. The Comprehensive Meta-Analysis Version 3-Free Trial (https://www.meta-analysis.com/pages/demo.php) was used for the meta-analysis. The extent of heterogeneity in the studies was assessed with the Cochran Q and Higgins I² tests and it was agreed that the I² rate exceeding 50% was an important indication of heterogeneity. Accordingly, when I² was greater than 50%, the Random effect results, and if the value was less, the Fixed Effect results were considered. The study data were composed of categorical variables and combined point estimates were calculated at a confidence interval (CI) of 95% for each result variable. All of the tests were calculated on a two-tailed basis and p<0.05 was accepted as statistically significant.
RESULTS
Searching results
At the beginning, the results of the scan yielded 732 records from the databases and 33 from additional scans, a total of 765 records. With the exclusion of the records that were repeats, the review carried out according to headings and abstracts yielded the full texts of 60 articles. From the review of the full texts, a total of 54 articles reporting the outcomes of pregnant women with COVID-19 and their infants were selected for the analysis (Figure 1).
Study characteristics
All of the studies (n=54) had been published in English. Twenty-two of the studies were case presentations, 13 were case series, 15 were retrospective (13) and perspective (2) cross-sectional studies, and 4 were case-control. It was noted that the study data had been collected over the period 8 December 2019 – 22 April 2020 and all were published in 2020. In 10 of the studies, no indication had been given as to the date of data collection18–27. The studies were from 15 different countries: China (34), United States (4), Iran (4), Italy (3), Peru (1), Columbia (1), Sweden (1), United Kingdom (1), Korea (1), Canada (1), Australia (1), Portugal (1), Turkey (1) and the Netherlands (1) (Table 2).
The studies contained data on 517 pregnant women with COVID-19 and 385 infants. The sample size of the studies varied between 1–118. It was seen that the age range of the pregnant women with COVID-19 included in the systematic review was generally between 21–45, that only one women was an adolescent (age 17 years)28, and that seven studies did not specify the ages of the pregnant women29–35.
Quality assessment results of the studies
The quality assessment scores in the case reports were Yes: 7/8 in 21 studies, and Yes: 6/8 in one study. The quality assessment scores in the case series were Yes: 10/10 in three studies and 9/10 in 11 studies. In all of the retrospective (13) and perspective (2) cross-sectional studies, the quality scores were Yes: 6/8, and in all of the case-control studies, the quality assessment scores were Yes: 7/10 (Table 2). A large majority of the studies reviewed met the criteria for quality assessment, representing a low risk of bias.
Results of the meta-analysis
COVID-19 symptoms in pregnant women
The pooled results of the studies reviewed in this systematic review and meta-analysis indicated that the symptoms of the pregnant women were observed to be high fever in 53% in 29 of the studies19,21,24,29-33,35,36,38-55 (Z=0.503, p=0.615) and cough in 40% in 28 studies19,21,24,29-31,33,35,36,38-43,45-56 (Z= -1.824, p=0.068). In fifteen studies, 21% of the pregnant women with COVID-19 had fatigue/tiredness/myalgia21,29,31,37-43,47,48,51,54,56 (Z= -9.034, p<0.001). Nineteen studies indicated that 21% of the pregnant women with COVID-19 suffered from dyspnea/shortness of breath19,21,29-31,38-44,46-48,51,54-56 (Z= -4.755, p<0.001) and 16% showed the symptom of sore throat in eight studies30,36-38,42,47,48,54 (Z= -4.613, p<0.001). In four studies, 16% of the pregnant women with COVID-19 had headache21,29,39,40 (Z= -2.954, p=0.003), 15% had chest pain/tightness in seven studies29,37,38,40,45,47,54 (Z= -8.496, p<0.001), and 13% had phlegm in three studies46,54,56 (Z=-3.028, p=0.002). In twelve studies, 8% of the pregnant women with COVID-19 complained of diarrhea29,33,36-39,41,46-49,55 (Z= -10.701, p<0.001), while 15% suffered from runny/congested nose in six studies21,42,46,52,56,57 (Z= -4.177, p<0.001). Three studies indicated that 12% of the pregnant women had chills/shivers37,38,57 (Z= -2.366, p=0.018), one study reported that 7 of 9 pregnant women had anosmia42 (Z=1.562, p=0.118), and another reported that 6 of 9 pregnant women complained of lethargy42 (Z=0.980, p=0.327). Furthermore, according to the pooled results of four studies, about 35% of the cases had been observed to be asymptomatic, but it was seen that this result was not statistically significant39,48,52,53 (Z= -0.568, p=0.570) (Table 3 and Supplementary file Figure 1).
Table 3
Meta-analysis results of maternal health
Diagnostic test results for COVID-19 in pregnant women
According to the pooled results of 31 studies, 80% of the pregnant women tested positive on the RT-PCR test19,21,24,29,30,32-57 (Z=9.424, p<0.001), and 88% had abnormal chest CTs in 21 studies30,32-40,43,46-50,52-54,56,57 (Z=8.097, p<0.001). In three studies, 37% of the women had abnormal chest X-rays24,42,44 (Z= -0.813, p=0.416). Moreover, in the pooled results of four studies29,34,49,52, 38% of the pregnant women had been diagnosed on the basis of clinical findings (Z= -1.029, p=0.303) (Table 3 and Supplementary file Figure 2).
Treatment methods using for COVID-19 in pregnant women
Fifteen studies taken into the meta-analysis showed that 87% of the pregnant women were treated with antibiotics29-31,33,38,43-46,48,50,52,54,55 (Z=3.236, p=0.001), and 75% were given antiviral treatment in 14 studies19,21,30,31,38,43,45,46,48-50,54,55,57 (Z=2.866, p=0.004, I2=60%). In five studies, 54% of the pregnant women were treated with hydroxychloroquine19,21,29,43,44 (Z=0.141, p=0.888), while 31% were treated with corticosteroids in six studies31,36,38,50,54,57 (Z= -1.227, p=0.220). In five studies, 88% of the pregnant women were treated with a Chinese medication (lianhua-gingwen)45,46,48,55,57 (Z=4.085, p<0.001). In two studies, 11% of the pregnant women were given intravenous immunoglobulin31,43 (Z= -3.985, p<0.001) and 8 of 9 pregnant women were given anticoagulant treatment in one study43 (Z=1.961, p=0.050). Again, 63% of the pregnant women needed oxygen support in 9 studies29,33,38,41,44,45,50,54,55 (Z=0.682, p=0.495), and 11% were intubated/placed on mechanical ventilation in 4 studies21,39,51,52 (Z= -1.450, p=0.147). According to the results of two studies, 77% of the pregnant women with COVID-19 had been treated and followed up as outpatients/by telephone29,37 (Z=0.829, p=0.407) (Table 3 and Supplementary file Figure 3).
This meta-analysis showed that in 2 studies it was reported that in order to prevent preterm labor, 15% were treated with hydration29,44 (Z= -1.177, p=0.239), and 6 of 7 pregnant women were given steroids in another two studies21,44 (Z=1.123, p=0.262), and 1 of 2 pregnant women were treated with magnesium sulphate (MgSO4) in one other study44 (Table 3 and Supplementary file Figure 4).
Comorbidities of pregnant women with COVID-19
The results of the meta-analysis indicated that 19% of the pregnant women in 4 studies had asthma21,24,29,42 (Z= -4.261, p<0.001), and it was reported in 3 studies that 18% of the women suffered from chronic hypertension21,29,30 (Z= -1.765, p=0.078). It was reported in 5 studies that 10% of the pregnant women had hypothyroidism31,43,50,53,55 (Z= -4.962, p<0.001), and 9% of the pregnant women had diabetes mellitus in three studies21,29,43 (Z= -4.813, p<0.001). It was found in 4 studies that 34% were obese21,24,43,44 (Z= -1.280, p=0.201), 34% had heart disease in two studies44,48 (Z= -0.317, p=0.751). It was reported in three studies that 9% of the pregnant women had Hepatitis B30,31,53 (Z= -5.347, p<0.001) and 17% of the women had polycystic ovary syndrome in three studies30,44,55 (Z= -2.803, p=0.005). In one study, 7% of the pregnant women were found to have thalassemia48 (Z= -2.550, p=0.011), and 1 of 4 of the pregnant women had cholecystitis in another study40 (Z= -0.951, p=0.341). It was noted in one study21 that 1 of 5 pregnant women had chronic kidney disease (Z= -1.240, p=0.2015), and 1 of 2 pregnant women had familial neutropenia in another study24 (Table 3 and Supplementary file Figure 5).
Additional conditions related to pregnancy
The pooled results of 11 studies in the meta-analysis showed that about 15% of the pregnant women had gestational diabetes mellitus24,29-31,37,41-44,48,56 (Z= -7.792, p<0.001), 6 studies indicated that 9% had hypertension29,31,38,42,44,53 (Z= -5.922, p<0.001), and 7 studies reported that 20% had preeclampsia30,33,37,38,44,52,56 (Z= -3.749, p<0.001). It was reported in one study that 2% of the pregnant women had cholestasis29 (Z= -3.694, p<0.001) and 16% of the pregnant women had anemia in another 2 studies37,40 (Z= -2.181, p=0.029). Another study revealed that 10% of the pregnant women had hypothyroidism37 (Z= -2.084, p=0.037) (Table 3 and Supplementary file Figure 6).
In another 2 studies, 8% of the pregnant women with COVID-19 exhibited reduced fetal movement29,40 (Z= -1.879, p=0.060), 2% of the pregnant women were in multiple pregnancy in another study39 (Z= -5.693, p<0.001), and 17% had early membrane rupture in 8 studies30,35-38,49,51,53 (Z= -6.260, p<0.001). It was reported that 11% of the pregnant women in 4 studies had placental abruption30,36,37,54 (Z= -3.980, p<0.001), 14% of the pregnant women in four studies had placenta previa36,40,48,54 (Z= -3.400, p=0.01), and 21% of the pregnant women in 2 studies had polyhydramnios/oligohydramnios36,54 (Z= -1.992, p=0.046) (Table 3 and Supplementary file Figure 6).
The pooled results in this meta-analysis showed that 8 studies indicated that 12% of the pregnant women diagnosed with COVID-19 were admitted into the intensive care unit21,29,30,35,41,44,51,52 (Z= -3.039, p=0.002), and 12% of the pregnant women in 2 studies were recorded as maternal mortalities39,43 (Z= -0.597, p=0.550). In one other study, it was noted that one of the two pregnant women had postpartum hemorrhage24. While the postpartum hemorrhage and maternal death results pertaining to the pregnant women with COVID-19 were not statistically significant, data related to admittance into the intensive care unit was statistically significant (Table 3 and Supplementary file Figures 7 and 8).
Results on pregnancy term and mode of delivery in pregnant women
Thirty studies examined in this systematic review and meta-analysis provided results on the delivery mode of pregnant women with COVID-1919,21,24,29-44,46,48-57. The pooled results of these studies were statistically significant in that 73% of the pregnant women with COVID-19 had delivered by caesarean section (Z=7.979, p<0.001). According to the results of 5 studies, 29% of the women undergoing caesarean section had a COVID-19 indication35,39,41,42,54 (Z= -1.872, p=0.061), and 43% of the pregnant women in 8 studies had undergone caesarean section due to obstetric indications29,33,37,39,41,42,52,54 (Z= -0.837, p=0.403). These results, however, were not statistically significant. It was found that 25% of the pregnant women in 17 studies went into preterm labor and this result was statistical significance21,29-33,35-39,42,45-47,51,54 (Z= -4.304, p<0.001) (Table 3 and Supplementary file Figure 9).
Perinatal results of newborns
In 15 studies examined in this systematic review and meta-analysis, 25% of the newborns of women with COVID-19 were born with low birth weight (<2500 g)19-21,24,30,31,35-38,42,43,46,54,55 (Z= -4.772, p=0.001). It was found that about 11% of the newborns in 26 studies had an APGAR score of <7 in the first minute19-21,24,29-31,33-40,42,43,45,46,48,50,51,53-55,57 (Z= -8.527, p<0.001). It was determined that 9% of the newborns had an APGAR score of <7 in the fifth minute in 27 studies19-21,24,29-31,33-38,40-43,45,46,48,50,51,53,54,55-57 (Z= -9.490, p<0.001). It was revealed that 23% of the newborns suffered fetal distress in 13 studies19,24,30,35-38,44,48-52 (Z= -4.591, p<0.001), and 4% of the newborns had fetal asphyxia in 10 studies31,35-39,47,48,53,55 (Z= -8.078, p<0.001) (Table 4 and Supplementary file Figure 10).
Table 4
Meta-analysis results on newborn health
In 11 of the studies in the meta-analysis, 28% of the newborns had been admitted into the neonatal intensive care unit (NICU) and this result was statistically insignificant20,29,31,33-35,40-42,45,49,50 (Z= -4.993, p=0.066). According to the pooled results of 12 of the studies examined, the neonatal death was observed in 6% of newborns and this result was statistical significance31,35-39,43,45,46,48,51,54 (Z= -7.411, p<0.001). It was found that the intrauterine death rate was 8% in a meta-analysis based on the results of 5 studies31,35,38,43,54 (Z= -4.564, p<0.001) (Table 4 and Supplementary file Figures 10–12).
Data on neonatal nutrition and care
In 9 studies examined in this meta-analysis, 36% of the newborns were breastfed by their mothers, who wore masks and strictly complied with hand-washing rules19,20,24,29,41,42,50,56,57 (Z= -2.911, p=0.338), and all of the eight newborns were fed formula in three studies19,20,40 (Z= 2.057, p=0.040). These results were statistically significant. Again, statistical significance was noted in 14 studies regarding the data that 88% of the newborns were isolated from their mothers19,30,32-34,40,42,44,45,47,49,50,54,57 (Z= 5.191, p<0.001). In 3 studies, 77% of the newborns were isolated together with their mothers, but this finding was not found to be statistically significant20,29,41 (Z=0.779, p=0.436) (Table 4 and Supplementary file Figure 13).
COVID-19 test outcomes of newborns
The consolidated results of 26 studies in this meta-analysis showed that 8% of newborns of women with COVID-19 the newborns tested positive in the RT-PCT test in the first 24 hours after birth19-21,29,31-39,41-43,45-47,49-51,53,54,56,57 (Z= -10.581, p<0.001), in 2 studies, 2 of 8 newborns tested positive for IgG19,34 (Z= -1.206, p=0.228), and 3 of 8 newborns tested positive for IgM in two studies19,34 (Z= -0.683, p=0.495) (Table 4 and Supplementary file Figure 14).
The consolidated results of eight studies in this meta-analysis revealed that 10% of the newborns tested positive on the RT-PCR test in the 48th hour and on day 14 postpartum19,20,30,31,33,37,40,47 (Z= -4.285, p<0.001). It was also seen in another 5 studies36,40,47,49,57 that 31% recorded abnormal results on the chest X-ray/CT (Z= -1.199, p=0.231) (Table 4 and Supplementary file Figure 15).
COVID-19 test outcomes in placenta, umbilical blood, breast milk, vaginal swab and amniotic fluid samples
In 8 studies examined, one of the placenta and the umbilical blood samples taken from pregnant women with COVID-19 in the first 24 hours postpartum was statistically significantly positive19,33,38,45,46,50,56,57 (Z= -3.916, p<0.001). On the other hand, samples of breast milk in 6 studies19,38,39,49,50,57 (Z= -3.691, p<0.001), of vaginal swab in 2 studies50,57 (Z= -1.647, p=0.100), and of amniotic fluid in 7 studies19,33,38,49,50,56,57 (Z= -4.158, p<0.001) did not test positive. In days 2–14 postpartum, 2 of 7 samples of breast milk were positive in 3 studies19,20,55 (Z= -0.900, p=0.368), and both of the two vaginal swabs were negative in one study17 (Z= -1.039, p=0.299) (Table 3 and Supplementary file Figures 16 and 17).
Pooled analysis results from case reports
According to the pooled results of the 22 case reports18,20,22,23,25-28,58-71, the most common symptoms among pregnant women recorded in case reports were fever (81.8%), cough (54.5%), fatigue/tiredness/ myalgia (40.9%) and dyspnea/shortness of breath (40.9%). It was seen that all of these pregnant women tested negative in the RT-PCR test and 72.2% had normal chest-CT scans; the method of treatment was mostly with antibiotics (68.2%) and antiviral drugs (59.1%). Of the pregnant women, 36.4% had underlying conditions and 36.4% had a history of preterm labor; 75% had delivered by caesarean. Eight mothers were admitted into intensive care and two mothers lost their lives. Six out of 20 newborns were admitted into the NICU, and only 2 were being breastfeed while 11 were separated and isolated from their mothers. It was seen that in 1 of the 13 infants tested in the first 24 hours after birth tested positive on the RT-PCR test and 1 of 3 babies tested positive for IgG and IgM. In the later period (48 hours –14 days), 2 of the 8 infants tested had positive RT-PCR results (Table 5).
Table 5
Pooled results of cases
| Outcome variables | Pooled n (%) | Outcome variables | Pooled n (%) |
|---|---|---|---|
| Data on maternal health | |||
| Symptoms (n=22) | Treatments (n=22) | ||
| Fever | 18 (81.8) | Antivirals | 13 (59.1) |
| Chills/shivering | 3 (13.6) | Antibiotics | 15 (68.2) |
| Cough | 12 (54.5) | Hydroxychloroquine | 3 (13.6) |
| Phlegm | 1 (4.5) | Corticosteroids | 8 (36.5) |
| Sore throat | 2 (9.1) | Anticoagulant | 1 (4.5) |
| Hoarseness | 1 (4.5) | Hydration | 1 (4.5) |
| Runny/congestion nose | 3 (13.6) | Tocolytic therapy | 3 (13.6) |
| Tiredness/fatigue/myalgia | 9 (40.9) | Antenatal steroid | 2 (9.1) |
| Dyspnea/shortness of breath | 9 (40.9) | Oxygen support | 6 (27.3) |
| Headache | 2 (9.1) | Intubation/mechanical ventilation | 6 (27.3) |
| Diarrhea | 1 (4.5) | Blood transfusion/iron supplement | 4 (24.1) |
| Asymptomatic | 1 (4.5) | Traditional Chinese medicine, supportive therapy | 1 (4.5) |
| Diagnostic tests (n=22) | Diseases related to pregnancy (n=22) | ||
| RT-PCR (+) | 22 (100) | Gestational diabetes mellitus | 1 (4.5) |
| Abnormal Chest CT (+) | 16 (72.2) | Gestational hypertension | 1 (4.5) |
| Abnormal Chest X-ray (+) | 6 (27.3) | Hyperemesis gravidarum | 1 (4.5) |
| Abnormal Chest USG | 1 (4.5) | Reflux | 1 (4.5) |
| Elevated IgG and IgM | 1 (4.5) | Preeclampsia | 1 (4.5) |
| With comorbidities (n=22) | 8 (36.4) | Preterm labor (<37 weeks) | 8 (36.4) |
| Comorbidities (n=8) | Multiple pregnancy | 2 (9.1) | |
| Diabetes Mellitus | 1 | Early membrane rupture | 2 (9.1) |
| Asthma | 1 | Admission to ICU (n=22) | 8 (36.4) |
| Hypothyroidism | 3 | Maternal death (n=22) | 2 (9.1) |
| Migraine | 1 | ||
| Obesity | 1 | ||
| Thalassemia | 1 | ||
| Duration of pregnancy and mode of delivery (n=20)* | |||
| Vaginal delivery | 5 (25.0) | ||
| Caesarean delivery | 15 (75.0) | ||
| Indication of COVID-19 for caesarean section | 2 | ||
| Indication of obstetric for caesarean section | 1 | ||
| Data on new born health (n=20)* | |||
| Low birth weight (<2500 g) | 4 | COVID-19 test results in postpartum period | |
| Apgar Score in the first minute <7 | 1 | The first 24 hours** | |
| Apgar Score in the fifth minute <7 | 1 | IgG and IgM (+) | 1/3 |
| Fetal distress | 1 | RT-PCR (+) | 1/13 |
| Fetal anomaly | 1 | 2nd–14th day** | |
| Admission to ICU of new born | 6 | RT-PCR (+) | 2/8 |
| Intrauterine death | 1 | Abnormal chest X-ray / CT | 0/2 |
| Neonatal death | 1 | Placenta and umbilical cord blood samples (+) | 0/6 |
| Breastfeeding | 2 | Breast milk (+) | 0/5 |
| Formula | 6 | Vaginal swab (+) | |
| Isolated separate from mother | 11 | Amniotic fluid (+) | 0/4 |
| Isolated with mother | 1 | ||
DISCUSSION
This systematic review and meta-analysis present the consolidated results of 54 observational studies, case reports and case series studies reporting the maternal and infant health outcomes during the pregnancy, birth and postpartum periods of pregnant women with COVID-19. The results of these studies are significant since they can contribute to improving the processes of treatment, follow-up and care services offered to pregnant women with COVID-19 and their newborns.
Outcomes of pregnant women with COVID-19
This systematic review and meta-analysis revealed that pregnant women with COVID-19 most commonly complained of high fever, chills/shivers, fatigue/tiredness/myalgia, cough, dyspnea/shortness of breath, chest pain/tightness, phlegm, runny nose/congestion, diarrhea, headache and sore throat. Similar results have been reported in previous systematic reviews concerning pregnant women5,6,72-75. In this study, it was found that COVID-19 was asymptomatic in approximately one-third of the pregnant women (35%). Lower percentages were reported in studies by Elshafeey et al.6 and Mullins et al.76 (7.5% and 32%, respectively). The results are similar to those observed in non-pregnant women77 and are valuable in terms of offering data that will be useful in the early diagnosis, follow-up and care of pregnant women.
It was found in this meta-analysis that a large percentage of the pregnant women with COVID-19 tested positive on the RT-PCR (80%), revealed an abnormal chest CT (88%) and that some women displayed abnormal chest X-rays (37%). The results of some studies show that a significant percentage of pregnant women with COVID-19 (38%) were diagnosed with clinical findings. Similarly, Smith et al.74 also report that approximately 90% of the women in their study were administered the RT-PCR test to confirm the diagnosis, that in 79% of the cases, tests were positive and RT-PCR negative in 23%. The authors reported that 99% of the pneumonia cases were diagnosed with CT findings. De Rose et al.5 state that the respiratory system samples taken from the women tested positive on the RT-PCR test in almost all of the women (99%). Elshafeey et al.6 report, however, that most of the women (90%) were tested with RT-PCR for a diagnosis and the infection was confirmed with radiological and clinical findings in a smaller percentage of women (10%). Della Gatta et al.72 report similar results. These data were similar with the diagnostic tests used with non-pregnant individuals77,78, indicating that more information is needed regarding the effects on pregnant women and infant health of diagnostic tests carried out with radiological methods.
It was noted in this systematic review and meta-analysis that pregnant women with COVID-19 were treated with antiviral drugs, antibiotics, intravenous immunoglobulin, a Chinese drug, hydroxychloroquine, corticosteroids, anticoagulants and oxygen support. Another study similarly indicated that pregnant women with COVID-19 were commonly treated with antibiotics, oxygen, antiviral drugs and corticosteroids73. It was reported in another study that a significant percentage of hospitalized pregnant women (28%) were put on oxygen support74. These reports show that pregnant women with COVID-19 were treated similarly to non-pregnant individuals78. There is a need for more data on the outcomes of such treatments on pregnancy and the infant.
In 2 studies examined in this systematic review and meta-analysis, a large majority of the pregnant women with COVID-19 were treated as outpatients or followed up by telephone. Again, it was reported in some studies with respect to preterm labor that hydration, steroid and MgSO4 therapy was applied to some of the pregnant women. These reported results are valuable in that they indicate that pregnant women with COVID-19 can be followed up with routine care similar to what would be implemented under normal circumstances.
This meta-analysis showed that some pregnant women with COVID-19 had underlying conditions such as diabetes mellitus, asthma, chronic hypertension, hypothyroidism, Hepatitis B, obesity, heart disease and polycystic ovary syndrome. On the other hand, Della Gatta et al.72 report no pre-existing comorbidities before the pregnancy. It is known that COVID-19 presents a life-threatening risk when there are comorbidities77 and that high-risk pregnant women with a history of chronic disease must particularly be carefully protected, making follow-up a matter of vital importance.
The study revealed that some pregnant women had diabetes mellitus, hypertension, preeclampsia and anemia. In a similar study, Della Gatta et al.72 reported that one of the 35 pregnant women had gestational hypertension while another suffered from preeclampsia. These conditions can be life-threatening for pregnant women with COVID-19 and necessitate cautious care and follow-up.
Furthermore, the study demonstrated that some pregnant women have additional conditions during their illness such as reduced fetal movement, multiple pregnancy, early membrane rupture, placental abruption, placenta previa and polyhydramnios/oligohydramnios. A previous study5 revealed similar results. These conditions may mean added difficulties in treatment, follow-up and care.
Outcomes related to the gestation period in pregnant women with COVID-19 and their mode of delivery
Our study indicates that preterm labor was observed in about 18% of the pregnant women with COVID-19. While this percentage is lower in the studies of Della Gatta et al.72 and Elshafeey et al.6 (12% and 15%, respectively), and De Rose et al.5, Smith et al.74 and Yang et al.75 report higher rates (34%, 64% and 21%, respectively). These rates are also higher than what is reported for the general population (11%)79,80. Preterm birth is closely associated with infant fatality and therefore this information must be carefully taken into consideration in the care and follow-up of infants with COVID-19.
This systematic review and meta-analysis reports that most of the pregnant women with COVID-19 (77%) were delivered by caesarean section. In some of the studies examined, caesarean childbirth was implemented mostly for obstetric indications (43%) and for some women for COVID-19 indications (29%). Elshafeey et al.6, De Rose et al.5 and Smith et al.74 reported similar caesarean rates in their studies (69%, 77% and 80%, respectively). It can be seen in other studies that the caesarean birth rate in women with COVID-19 is much higher72,73,75. Although these outcomes have not been reported as indications, it is apparent that the caesarean birth rate among pregnant women with COVID-19 is markedly high. This outcome should be assessed in terms of maternal and infant health.
Perinatal outcomes of the newborns of pregnant women with COVID-19
It was noted in this systematic review and meta-analysis that 19% of the newborns of women with COVID-19 was of low birth weight. A previous study revealed a similar percentage5. Contrary to this data, however, Elshafeey et al.6 and Yang et al.75 report a lower frequency of low birth weight (8% and 5.3%, respectively) while Smith et al.74 point to a higher rate (43%). These results can be associated with the incidence of preterm labor among pregnant women with COVID-19.
It was found in this systematic review and meta-analysis that some infants born of pregnant women with COVID-19 had low APGAR scores (<7) in the first (4.3%) and fifth minute (4.6%), suffered from fetal asphyxia and fetal distress (14%). The incidence of fetal distress was reported as lower in studies by Elshafeey et al.6 and Yang et al.75 (8% and 11%, respectively). De Rose et al.5 reported this rate to be 22%. In Smith et al.74, it was reported that all the newborns in their study exhibited normal APGAR scores. Again, in the study by Yang et al.75, a similar outcome was reported with regard to neonatal asphyxia (1.2%). These results are significant in that they indicate that infants born of women with COVID-19 must be kept under close scrutiny in the perinatal period.
In some studies that were reviewed for this systematic review and meta-analysis, there were data that indicated that some newborns were being breastfed (33%). Elshafeey et al.6 had presented similar results in their systematic review. These data are valuable in terms of the health of breastfed infants and indicate the need for additional research and the adoption of urgent measures to develop methods by which women with COVID-19 can safely provide their infants with the breast milk they require.
It was seen in the meta-analysis that some newborns of women with COVID-19 were administered various tests in the first 24 hours and later after birth. The analysis showed that some infants tested positive in the first 24 hours and some in the period Day 2 – Day 14 on the RT-PCR test. It was also seen that some infants displayed positive IgG and IgM results in the first 24 hours. It was found in the meta-analysis that the samples taken from pregnant women with COVID-19 (placenta, umbilical blood, breast milk, vaginal swab and amniotic fluid) tested positive on the COVID-19 test in the first 24 hours in only one woman in the placenta and umbilical blood specimens and in two women in Days 2–14 in the breast milk specimens. Similar results can be seen in other studies as well5,6,72,74. These results are not sufficient, however, in terms of making an assessment of the possibility of vertical transmission; more evidence is needed.
Outcomes on admittance of pregnant women with COVID-19 and their infants into the intensive care unit and maternal–infant death
It was noted in this systematic review and meta-analysis that a significant portion of the pregnant women were admitted into the intensive care unit and were administered intubation/mechanic ventilation. Elshafeey et al.6 similarly reported that some women were admitted into intensive care (4.4%) and were administered mechanical ventilation (1.6%). In the study by Smith et al.74, it was reported that one woman (4.3%) needed mechanical ventilation and was admitted into the intensive care unit. These results indicated that COVID-19 is a serious health issue that threatens maternal/infant health during the period of pregnancy and beyond.
In all of the studies reviewed for this systematic review and meta-analysis, nine mothers were reported to have lost their lives. In their study of 108 pregnant women, Zaigham and Andersson73 reported no incidents of maternal mortality. Although the percentage was higher than the general maternal mortality rate, it is lower that the mortality rate reported for SARS-CoV-2 (3.8%)81.
It was again seen in this study that a significant portion of the newborns were admitted into the NICU. Similarly, in some other previously published systematic reviews, it was reported that some infants had been admitted to the neonatal intensive care unit due to an additional symptom or for further care and follow-up6,74. These results may be associated with the high level of premature birth and low birth weight rates.
In all of the studies reviewed for this systematic review and meta-analysis, it was reported that 3 out of 385 infants lost their lives in the intrauterine stage and 5 died as neonates. Smith et al.74 also reported in the data they presented for 37 infants that one baby had died in the intrauterine and one in the neonatal stages. Moreover, Yang et al.75 and Zaigham and Andersson73 reported one mortality in the intrauterine and neonatal stages in their studies. In the systematic review of Elshafeey et al.6, the authors provided findings from 256 newborns, reporting two stillbirths and one neonatal death. Both De Rose et al.5 and Della Gatta et al.72 reported one stillbirth in each of their studies. The rates are higher than in general infant rates and are important in that they indicate that COVID-19 is responsible for increasing infant fatalities.
Strengths and limitations
The high score noted in the current quality assessment of the studies examined in this systematic review and meta-analysis and the wide range of additional resources available for scanning constituted the strengths of the study. Also, the large number of pregnant women and infants whose data were reviewed in this systematic review was another strong point that consequently strengthened the conclusions that were drawn. The study was further strengthened by the fact that the results were based on reliable methods of analysis, the subject matter was examined from different aspects, and the results attained were supported by outcomes reported in previous studies. It can be said, however, that the low extent of homogeneity in the studies reviewed may have weakened the power of the evidence. To keep this factor under control, the Random Effect model was preferred in analyses in which the extent of heterogeneity was high. Other limitations were that only studies published in English were taken into the analysis, most of the studies were based on small sample sizes and were derived from Chinese sources, China being the location where COVID-19 had originated, which also meant that studies published in Chinese and other languages could not be included.
CONCLUSIONS
We tried in this systematic review and meta-analysis to uncover comprehensive data on the status of pregnant women with COVID-19 and their infants, based on the results of 54 studies. The evidence gathered showed that the symptoms, diagnosis, treatment and comorbidity factors associated with COVID-19 were the same in pregnant women as in non-pregnant women. It was, however, noticeable that morbidity, preterm and caesarean birth rates necessitating admission to the intensive care unit as well as maternal and perinatal death rates were higher in pregnant women with COVID-19 and their infants. It was also found that some babies tested positive in the first 24 hours and on Days 2–14 in the RT-PCR, IgG and IgM tests. An important part of these results is also supported by previous systematic review and meta-analysis studies9,10.
Based on existing data, health professionals play a key role in protecting and improving the health of pregnant women during a pandemic. Besides tending to the needs of the other members of the community in this time, these health professionals must take preventive and risk-reducing measures in their care of the high-risk group of pregnant and possibly pregnant women to ensure that no contact is made with the SARS-CoV-2 infection. Moreover, they need to take into consideration the individual characteristics of each pregnant woman in their effort to protect and improve maternal and infant health, basing their actions on the guidelines to care and follow-up set forth by international organizations. It is necessary also in this period to take the measures that will ensure the prevention of neonatal infection and to develop guidelines that will allow infants to benefit from breast milk. Additionally, scientific studies are needed that will explore the long- and short-term effects of the infection and the impact of care and follow-up services specifically adopted for use during the pandemic on maternal and infant morbidity and mortality.